It’s always a little strange to get back into regular writing after even a short period of doing something else. There’s some very specific brain muscles that get developed when you write on a daily basis, and I think they tend to atrophy shockingly quickly. But it’s still healthy to take an occasional break and do something else, even if just for a few days, and I’m pretty sure I did just that. I’m going to tell you a bit about what I did, even though it doesn’t really involve cars. So don’t tell David.
There’s a public art festival around where I live called Uproar, where artwork is made by selected artists and then distributed around the area in public places. I’m a big fan of public art and think events like this are ideal for reminding people just how much better life is when you throw a bunch of art at it.
Anyway, this year, about a day before the deadline, I decided to enter a proposal, despite being way too busy and not really certain I’d be actually able to pull it off. Still, I assumed that my chances of getting chosen were fairly low, so what could it hurt?
Well, the selection committee must have had a moment of pity or a mild, collective stroke, because they accepted my idea, which was great save for the fact that I had to actually, you know, do it. The idea I came up with was one based around a favorite medium: repurposed arcade video game cabinets.
The concept was to take an arcade machine and make it a medium for something that was about as far from a video game as possible: live plants. Here was my initial sketch:
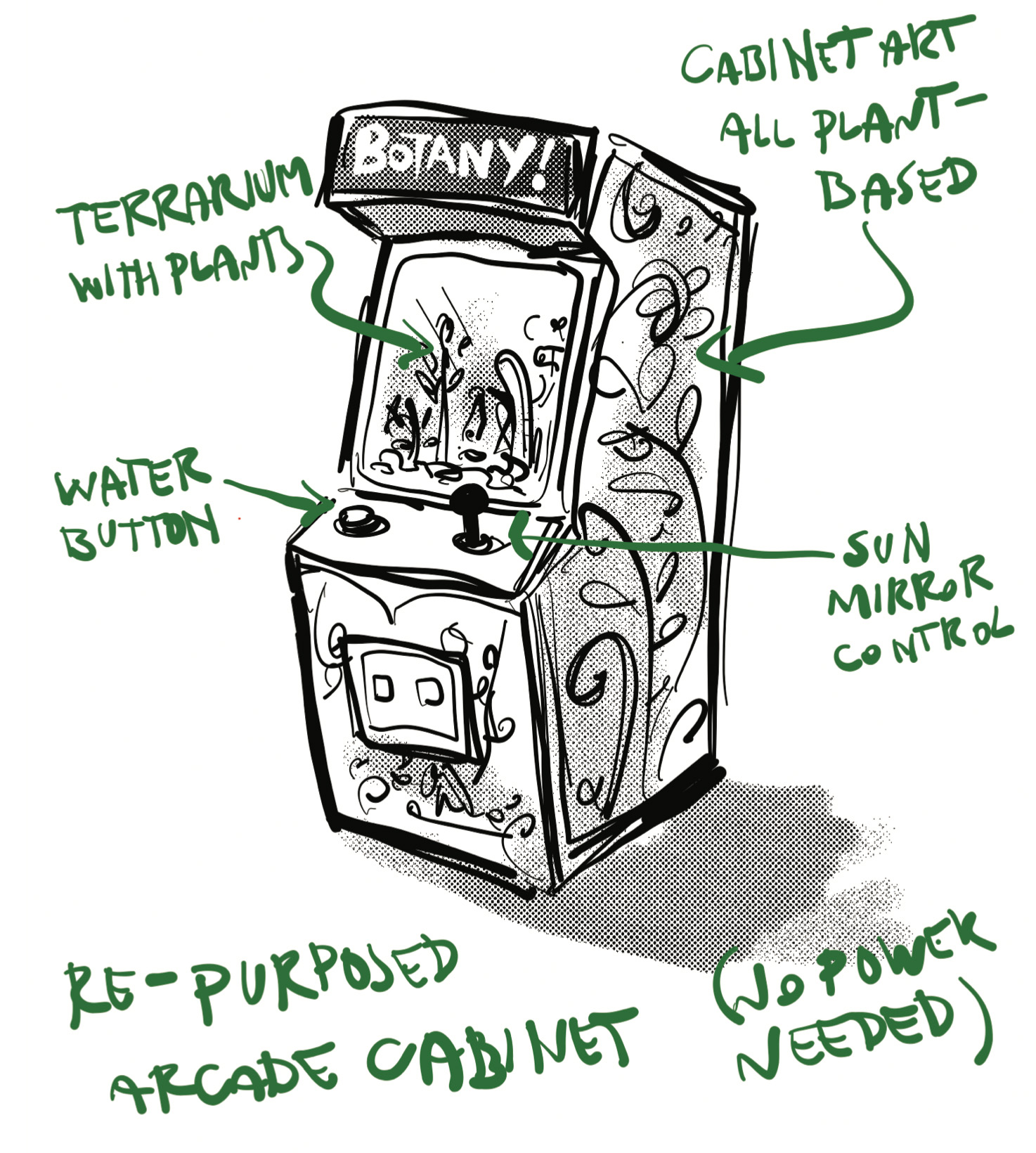
So, basically, the plan was to replace the screen of an arcade machine with a terrarium full of plants, but keep the interactive nature of an arcade game by providing controls for watering and giving the plants sunlight. I changed the joystick-operated sun-mirror-whatever to a far more manageable button for some grow lights, and retained a button that operated a pump to spray/drip water onto the plants.
Here’s it in action:
I think I managed to stay pretty close to my initial vision, I’m happy to say. The whole thing runs on a car battery, so there’s a car tie-in, and this was a big impetus for me finally getting the flywheel fixed in my ’89 F-150, since I’m finally sick of starting the damn thing with a wrench.

I had the clutch replaced, too, and now it shifts smoothly and starts without having to roll under the car! Just like a Bugatti Veyron! It’s no different, except the F-150 can carry a whole arcade machine and a Bugatti can’t.

The cabinet started life as an Atari Tempest cabinet, and was then converted to be a Golden Axe cabinet at some point, complete with this deeply hilarious marquee art:

What is that dude riding? Is that some kind of griffin? A hawk-bear? And that dragon looks like he should have a piano keyboard under those claw-hands.

I replaced all the art and controls, and now there’s just HYDRATE! and PHOTOSYNTHESIZE! buttons, though you can also breathe your own CO2 onto the plants for a bonus.

I know next to nothing about plants, but I think the terrarium plants look pretty good! There’s even an orchid in there!
How long do we think that car battery will last? How many times a week will I need to refill that gallon-sized water tank? I have no idea right now. I just hope people like it and have some fun with it. If you’re around Hillsborough, NC, then please feel free to take a little walk to the Thomas Stevens Gallery, and try it out. Give it light and water! Experience hardcore vegetative action! You don’t even need a quarter!
I’ll get back to car-focused content, of course, but I was just excited that I actually managed to get this thing done in time, and I wanted to show all of you. Thanks for indulging me!









Great job ! It’s really creative. I especially like the fonts used, I’m not sure what they’re called but I associate them with the ones used in SpongeBob. It’s funny how a font can evoke a memory like that.
That dragon is actually pretending to be Journey’s Jonathan Cain playing air keyboard in the Separate Ways video.
That’s so meta considering that Journey had their own video game.
Love it!
Introduce some worms! They’re a critical part of the ecosystem and would look great up against the glass.
Or some mealworms. They’ll go through the hole worm-pupa-beetle-egg lifecycle.
Yum!
Fun fact: Ther are few worms in Florida because the soil is so weak and it’s so hot.
They’re all at a club in Palm Beach
LOL!
I just have one note here and it says “no notes!”. I love the terrarium as a piece.
Ah. I see you really did stop paying attention to video games after the 1981. You missed out on a lot of great 80’s arcade action.
Very very cool. If you ever do another you could use an old claw game mechanism with a grow lamp suspended instead of a claw. “Which plants are good and deserve more light? Which plants defied your will and deserve punishment?” Might be good for an installation in a Redder part of NC.
Or maybe instead of a grow lamp, use an arrangement of mirrors to direct light onto the area of the player’s choice, moved around by the claw mechanism.
I think my favorite part is the exclamation point. The entire cabinet is objectively excellent, of course, but that little piece of punctuation conveys excitement! and puts it over the top. 😀
It’s good to exercise different mental muscles. Most of my days are spent with technology projects, and getting into the shop and futzing around with tools and materials is a welcome gear change.
At the moment I have a bruised right foot from trying to kickstart a recalcitrant 44-year-old motorcycle and I’m working on a design for an hat.
The punctuationis great, very gameshow. Very Jeopardy!.
Love it!
That is a fantastic project, and I’m glad you shared it. I really like the initial idea of a joystick-controlled mirror, but I think the grow light solution is good for broader display purposes. I wish I were local to check this out!
Thank you! This creation makes my day!
Also: I have done the math. In our corner of the metaverse, the chance of another artist powering another arcade cabinet rebuild with a car battery? Less than 10 to the minus 29th!
Also also: Peter, Matt, the comment section would be so much better if it supported scientific notation! Please help!
Art and science together in one sweet package – I love it! As a chemist and educator, I’m always happy to see science and art team up to educate and entertain and enhance society.
This is awesome!
Jason, I don’t think ANYONE here would be bothered by another article detailing the build… Although it’s likely too late for that, since I’m assuming this barely came together in time just creating it, much less documenting each step with photos. I cannot help but think that this feels like a modern work by one of my heroes, The Secret Life of Machines’ Tim Hunkin… https://www.timhunkin.com/
I barely have a DVD player anymore, but I treasure my signed DVDs of his series.
Tim Hunkin who among other things built the flying pigs for Pink Floyd’s Animals tour.
Might want to get a current one while you can.
Software updates block many newer discs from playing on older decks.
If you haven’t seen it, Tim did a series on YouTube in recent years. “The Secret Life of Components” about the various bits he uses to make his contraptions. Features a lot of video of them, including the political art arcade games at his arcades in London.
Cool Beans
I love this, and I’m glad the cabinet wasn’t sacrificed while still containing Tempest, which was my go-to in the student center basement at college. If you ever have to cheat and replenish the terrarium with some high-quality fake plants, I won’t tell.
I love this piece, but if you ask me, the terrarium desperately needs some beetles, such as Eudicella sp.
It’s usually unwise to introduce bugs into a video game/computer system. But I get where you’re coming from.
Maybe some artwork of some beetles, perhaps a yellow one, and a gray one that’s upside-down?
The plants are happy but that truck is screaming out in agony for some soap and a bucket.
I know! it’s been so gross and rainy here nonstop. That truck needs a bath, badly
Moss truck. You know you want to.
Stealth Gauntlet reference or are my 80’s arcade memory neurons just overactivated right now?
I like washing cars in the rain, it means I can skip the rinsing and drying stages.
Why not make the F150 a mobile companion piece to “Botany!” And just slap on some graphics that say “Sloth Fur Ecosystem!”
Otto to the rescue!
That is a great concept, and completed brilliantly!
Although you should have powered the water spray with the 2CV manual washer pump.
You beat me to it – incorporating archaic French automobile features is how you justify posting about art around here!
That’s awesome and great execution! The graphics make it look like something you’d find as an interactive museum display.
I have some houseplants that I’ve kept alive for years by doing almost nothing, so I’m not a plant expert by any means, but as it’s something I’ve done wrong myself, I’d be concerned that people could overwater them. Maybe a misting nozzle so less water is released? Hopefully, some plant people can chime in.
Golden Axe is one of THE GREATEST video games ever made. The screams of the children, hopping on one of those swing-tail dudes, or one of those fire-breathing dragons. And the 8-bit music that played the entire time.
I think I always played as the elf-with-axe guy.
We had one in the basement of my college dorm. So yeah so much laundry NOT done with my quarters. The goal became complete the game on a single quarter.
IIRC, the dwarf had a hellacious summon lightning from the sky attack.
Yup, plus he was just the fastest. I also played as the Valkyrie, because she had good kicks.
That side-to-side shoulder move against those big red Roman things- wow.
Looks great! After its stint at the gallery, you can put it in the Wooden Nickel for us low-brow types.
Super cool, and I would be jazzed to find such a thing in the wild. These sorts of art pieces are what make downtown areas so much more engaging and fun than endless strip mall hell.
And taking a look, Hillsborough seems like a pretty nice little area.
That’s was a great idea and beautifully executed. It’s looks factory!
That’s awesome. My friend works in Hillsborough and is also an artist (painted the facade of a business in town); I’ll tell him to check it out and get photosynthesizing!
Amazing idea! I also like the formula. So many people don’t know that plants destroy water, and animas (including humans) create water!
Unfortunately, these people will probably not understand the chemistry behind it either.
It’s the circle of life. We feed the earth, the earth feeds us. Too many short-sighted people forget the first part and only think about the second.
Chemist here. Not really true, Martin – you might have meant “carbon dioxide” rather than water? Neither animals nor plants create or destroy water, but both use water in various ways. Animals do produce carbon dioxide, though, which plants use to create carbohydrates and oxygen through photosynthesis, so there is some great teamwork going on between animals and plants. The amount of water in earth is basically fixed (although we do lose a bit each year by losses into space and through photolysis in the upper atmosphere).
Both plants and animals use chemical reactions that use water. That’s why we both need a steady intake of water, or we’ll die fairly quickly. We both use water to produce various chemical compounds such as proteins. And also in cellular structures and as the medium for most chemical reactions that take place inside of us. And both plants and animals utilize chemical reactions that produce water, as well, by breaking down other molecules, such as carbohydrates.
https://www.scientificamerican.com/article/how-did-water-get-on-earth/
https://en.m.wikipedia.org/wiki/Origin_of_water_on_Earth
https://bns.institute/applied-sciences/key-properties-uses-water-biochemistry/
“Chemist here. Not really true, Martin – you might have meant “carbon dioxide” rather than water? Neither animals nor plants create or destroy water”
Huh? Water is just as much destroyed as a reactant in photosynthesis as CO2 and created just as much as a product as CO2 in cellular respiration.
I think we have a different definition of “destruction”. Water is rearranged but not irreversibly. It’s still all there and can be re-generated by another process. If that’s what you and Martin mean by “destroyed”, then that’s fine, but I think of it as meaning to render it permanently changed or eliminated beyond recovery. In that sense, some water is destroyed by photolysis in the upper atmosphere when UV radiation causes it to become hydrogen and hydroxide or oxygen (depending on the processes involved) and some of the hydrogen is lost to outer space. Then, the water it came from has been destroyed for all practical purposes.
This is the same argument for “created”. We don’t create water or CO2. We produce it by rearranging other molecules. Some of that CO2 is then rearranged to form carbohydrates in plants through photosynthesis.
You might as well say you had “created” a lego structure by rearranging the legos. You only built it, you didn’t create it (from nothing).
Anyway, perhaps it’s just semantics but I like to try and correct any chemical misapprehensions I see. If people believe that water can be “created” by animals or anything else, they might not take water conservation efforts seriously. And they should because, apart from a few ice comets that might arrive on the planet, we’re not getting any more water on this spinning rock.
By your logic a book is not “destroyed” by fire, merely rearranged into smoke, ash, water and soot. A recreation of that book however is not the exact same physical object as the original book.
“Water is rearranged but not irreversibly.”
No. It is no longer water. It is destroyed, not”rearrainged”.
You can recreate it IF you put the exact same atoms back in the exact same bonds but thanks to entropy that never happens outside a thought experiment. You may as well hold your breath anticipating all the air in the room you are in suddenly concentrating in one corner, leaving you in a perfect vacuum.
In the real-world what happens is the atoms of that destroyed molecule of water are dispersed to maybe, eventually form bonds with different atoms to make a completely new and different molecules of water.
“You might as well say you had “created” a lego structure by rearranging the legos.
And in saying so one would be correct. In that scenario the builder did indeed create a Lego structure by rearranging the legos.
“You only built it, you didn’t create it (from nothing).”
You built it AND if its an original design you created it as well. If it’s not original you recreated someone else’s work.
There was no structure in the original pile of legos, only a pile of legos. So the structure was (re)created from a random pile of legos. What you are talking about is the creation of legos which is another matter entirely from creating a structure out of individual legos.
“I like to try and correct any chemical misapprehensions I see.
I applaud your enthusiasm but in this case you are creating chemistry misinformation, not destroying it.
” If people believe that water can be “created” by animals or anything else, they might not take water conservation efforts seriously.”
That’s the wrong approach. Water conservation isn’t about conserving the water in seawater, sewage, the earth’s mantle, ice, steam or in clouds. Water conservation is about preserving fresh, potable water where its available to be used by life.
And they should because, apart from a few ice comets that might arrive on the planet, we’re not getting any more water on this spinning rock.”
It’s not about getting MORE water on this spinning rock it’s about making sure we have enough liquid water without too much other stuff like salt, dirt, microorganisms, dangerous levels of radioactive isotopes, etc and that water is to be had where it is needed.
Where did you say you got your chemistry credentials again?
“By your logic a book is not “destroyed” by fire, merely rearranged into smoke, ash, water and soot. A recreation of that book however is not the exact same physical object as the original book.”
Not so, Bro! (all such appellations are not intended to imply or assume gender) Although biological digestion of chemical compounds is sometimes referred to as “slow burning”, that is accomplished through reversible equilibrium processes, whereas destruction by fire is a non-reversible, non-equilibrium process, so it’s not a good analogy at all.
As for the “destroyed” vs “rearranged” and “created” vs “produced” arguments, I admitted it may be just semantics, but I think the details are important. “Destroy” and “create” are commonly used to refer to “annihilating” and “producing from nothing” (as in the phrase”God created the heavens and the earth”). Animals do not “create” water. Stop drinking water for a few days (please don’t do this!) and eat all the food you want and see how much water you “create”. You won’t be producing much water, either. If we “created” water by digestion, we wouldn’t have to constantly drink it. Some water is produced through chemical rearrangement of the atoms in the materials we intake (through breathing and eating), but we aren’t “creating” anything. And we aren’t net producers of water, either. We are net users of water. The demands of the chemical processes in our body far outstrip the small amount of water produced through digestion, hence the need for constant intake of water.
“Water conservation isn’t about conserving the water in seawater, sewage, the earth’s mantle, ice, steam or in clouds. Water conservation is about preserving fresh, potable water where its available to be used by life.”
Where do you think the fresh, potable water we need comes from? It comes from all those sources you mentioned. They are all part of the hydrologic cycle and you can’t conserve one without conserving the others. That’s my point, the water supply on this planet is a closed system (with a few leaks into space) and what we’ve got is all we’ve got. If people start believing we can “create” water, they might be less likely to conserve what we have.
“I applaud your enthusiasm but in this case you are creating chemistry misinformation, not destroying it.”
Au contraire, mon frère! I am merely trying to make some of these comments about “creating” or “destroying” water more accurate. I won’t even bother to respond to your uninformed nonsense about “the exact same atoms”, etc.
“Where did you say you got your chemistry credentials again?”
I have a Ph.D. in chemistry from the University of Denver and I have taught chemistry at the college/university level since 1996. What are your chemistry or science credentials?
What are your chemistry or science credentials?
B.S. Biochemistry and Ph.D. in Physical Chemistry from the University of California. I have taught chemistry at the college/university level too. And yet I still disagree strongly with your arguments.
Although biological digestion of chemical compounds is sometimes referred to as “slow burning”, that is accomplished through reversible equilibrium processes, whereas destruction by fire is a non-reversible, non-equilibrium process, so it’s not a good analogy at all.
It works in that *something* no longer exists in it’s previous form. The book is no longer a book, just as the water is no longer water. In that regard the analogy is sound.
The reversibility of the biological process is irrelevant. The products are dispersed, never to meet again. The original water molecule is gone forever, e.g. it has been destroyed. That destroyed molecule will be replaced by a new molecule of water made from different atoms somewhere else but its not the same molecule.
Animals do not “create” water. Stop drinking water for a few days (please don’t do this!) and eat all the food you want and see how much water you “create”.
We humans drink water because our cooling system is based on the evaporation of water and because our waste systems uses a lot of water to solvate our wastes.
Other animals which have evolved to a less water rich environment do not drink water and they get by just fine:
https://enviroliteracy.org/what-animal-cant-drink/
“Where do you think the fresh, potable water we need comes from? It comes from all those sources you mentioned. They are all part of the hydrologic cycle and you can’t conserve one without conserving the others.
If you believe that then why bother with water conservation at all? Seawater, sewage, clouds, mantle, lakes, cellulose, or in bottles it’s all water right?
That’s my point, the water supply on this planet is a closed system (with a few leaks into space) and what we’ve got is all we’ve got.
Short of building a giant space wall there is absolutely nothing we can do about that.
Au contraire, mon frère! I am merely trying to make some of these comments about “creating” or “destroying” water more accurate.
OK:
Ripping a molecule apart into its constituent atoms fits every one of those definitions.
Assembling a molecule out of constituent atoms is to bring that molecule into existence. “Produce” is also applicable here:
producetransitive verb
a : to cause to have existence or to happen
b : to give being, form, or shape to
As was also demonstrated with metabolic water
As I said a couple of times already, it may just be semantics, but I think the words “use” and “produce” are more accurate to refer to these processes. While there are edge cases, the vast majority of animals use far more water than they produce. Tell me this, fellow prof, if you needed to produce water in quantity, would you a) dig a well, b) set up a desalination system or c) raise a bunch of cows? What, you wouldn’t choose c? But animals “create” water, don’t they?
“If you believe that then why bother with water conservation at all? Seawater, sewage, clouds, mantle, lakes, cellulose, or in bottles it’s all water right?”
Yes it’s all water and we need to conserve all of it and make sure we can clean it up whatever we need it. And, to your point, whenever we can avoid contaminating already potable water, we should. But to my point, spreading the message that “animals create water”, is factually untrue and potentially dangerous.
And I strongly disagree with your statement that reversibility is irrelevant. Martin’s and your insistence that “plants destroy water” ignores the fact that almost all of the water used by plants to produce hydrocarbons is returned to the environment eventually, and mostly through the decomposition of plant matter. This is only possible because the processes used to rearrange those water molecules into other forms are reversible. And, as I said before, your comment about identical atoms making up a molecule is incredibly uninformed, especially for someone with a PhD in chemistry. Let’s take a glass of pure water, for example. Do you know that the water molecules in that glass exchange hydrogen atoms constantly? (https://www.pnas.org/doi/10.1073/pnas.1306642110)
By your logic, water “destroys” water because, after some period of time, none of the original water molecules consist of the “exact same” atoms.
Tell me this, fellow prof, if you needed to produce water in quantity, would you a) dig a well, b) set up a desalination system or c) raise a bunch of cows? What, you wouldn’t choose c? But animals “create” water, don’t they?
If I wanted to produce large amounts of water I would indeed dig a well or set up a desalination system but I would do so based on this definition:
Not this definition:
https://www.merriam-webster.com/dictionary/produce
But to my point, spreading the message that “animals create water”, is factually untrue and potentially dangerous.
And I say denying ‘”animals create water”, is factually untrue as I have already shown “animals create water” to be factually true.
If you choose to use the word “produce” because you feel its more accurate nobody is going to fault you for that. But to deny others the correct usage of “create” in that context is potentially dangerous.
“Do you know that the water molecules in that glass exchange hydrogen atoms constantly?”
Of course! Every high school chemistry student knows that.
Those high school students also know what is happening is water molecules lose and gain protons to form negatively charged hydroxyl ions and positively charged hydronium ions.
By your logic, water “destroys” water because, after some period of time, none of the original water molecules consist of the “exact same” atoms.
Those ions have different masses, spectral, electrical and chemical properties than the original water molecules so yes,by all measures water is destroyed and new ions are created.
Those students also know that at the same time those molecules of water are being destroyed to create ions other hydronium and hydroxyl ions are destroyed in the reverse process to create new molecules of water. The constant destruction and creation of molecules and ions is perfectly balanced in a macro scale equilibrium. The sheer number of atoms in the solution ensures vanishingly few of the original water molecules present at time zero are ever recreated. The very few which are will eventually be destroyed again in the same process.
So what?
Water is “destroyed” when the bonds between the hydrogen and oxygen atoms is severed. It is no longer water; it is now hydrogen and oxygen, and the atoms may then combine with other atoms to form different molecules (which does happen during photosynthesis; it forms biomass, essentially). The water no longer exists; it’s gone.
If the biomass is later “burned”, either by literally burning e.g. wood, or by an animal (including a human) metabolizing it, water is “created”. There’s water again, but it’s not the same water; now different oxygen and hydrogen atoms are bonded together to form water molecules.
If you build a wall of LEGO bricks, you have a wall. If you then go and dismantle it by pulling the bricks apart and throwing them all into a box with your other LEGO bricks, do you still have a wall? No. It is destroyed. Do you still have LEGO? Sure. If you then go and built another wall from these or other bricks, do you have a wall again? Sure, but it is not the same wall: it is made of different bricks in different positions (the chance of re-building it exactly the same way is very small). It is a new wall that you have created.
Thanks for your response, Martin. I apologize for any imprecision in my own language or my clunky analogy of legos, but I have tried to explain more completely in my response to Cheap Bastard, above. My disagreement with your comments is due to the common use of the words “created” and “destroyed”. I believe it is more precise to use “produced” and “used” as they don’t imply any kind of absolute creation (from nothing) or destruction (to nothing). But more importantly, animals are not net producers (or creators) of water. Sure some water is produced through biological processes, but animals use far more water than they produce. So your comment about animals creating (or producing) water is kind of nonsensical. Animals are, however, net producers (creators, if you prefer) of carbon dioxide, which plants also use to create carbohydrates.
The word “use” is very dangerous when applied to water, because it mostly means something entirely different. When you flush the toilet, you don’t use water; you only use fresh, clean water. The water stays water. It just has additional, unwanted stuff in it so it is no longer clear and fresh, but it is still water.
That’s why it is so important to use “create” and “destroy” when you mean chemical processes (nothing is created from nothing nor destroyed into nothing). Fresh, clean water is “produced” in water-purifying facilities that take ground and/or surface water and turn it into drinking water. Desalination facilities do the same with salt water. But again, it is water coming in and water going out; it only is now safe to drink, which it wasn’t before.
Humans use fresh water: for drinking, for example. But the water a human drinks will be emitted as water again: part of it will become urine, another will become sweat and evaporate. Some may leave the body as blood (not preferred). Some will become part of the body, but even that is still water (humans are about 70% water). None of that is chemical; these are all physical processes.
Chemically speaking, humans (as all animals) are net creators of water: more water will (eventually) come out than goes in. (I say eventually because some of the water will only be released after the human is dead.)
Incorrect. Quite a lot of the water that is taken in by animals will be bound up in other chemical compounds and will not be released as water when the organism dies. If the organism is cremated, some of that bound water will be released, but most organisms are not cremated. An animal is not a net producer (“creator” in your chosen balance) of water.
Quite a lot of the water that is taken in by animals will be bound up in other chemical compounds and will not be released as water when the organism dies.
If that were true the world would be covered with billions of years worth of dead animal bodies.
It is. It’s called “humus” or “dirt”. I didn’t say the animals don’t break down and degrade – they do. But they also don’t dissolve into a puddle of water. Some of that water was used to create chemical compounds in the body and when the body decays, it may or may not be released as liquid water.
It’s called “humus” or “dirt”.
Which is made by the process of decomposition:
Decomposition is the process by which dead organic substances are broken down into simpler organic or inorganic matter such as carbon dioxide, water, simple sugars and mineral salts.
https://en.wikipedia.org/wiki/Decomposition
Those simple sugars become food for other organisms and are metabolized into liquid water.
In turn that “humus” or “dirt” in turn becomes dust. How? By releasing the water as vapor which in turn becomes liquid water.
An animal is not a net producer (“creator” in your chosen balance) of water.
They are indeed:
Metabolic water refers to water created inside a living organism through metabolism, by oxidizing energy-containing substances in food and adipose tissue. Animal metabolism produces about 107–110 grams of water per 100 grams of fat,[1] 41–42 grams of water per 100 g of protein, and 60 grams of water per 100 g of carbohydrate.
https://en.wikipedia.org/wiki/Metabolic_water
Which means 70% of the fat, protein and carbohydrate in animal feed is used to create water. That’s a LOT of water.
Yep, but not nearly as much as you drink every day. I don’t think you understand the idea of a “net producer”. We use far more water than we produce. But if you don’t wish to understand that, then there’s nothing I can do about it.
If you limit the definition of “water” to only fresh, potable liquid drinking water then perhaps sure, animals consume more fresh, potable liquid drinking water than they produce.
If you use the very strictest definition of “water” to mean only ultrapure >18.18 MΩ·cm water then animals are a null. They neither consume nor produce such pure water.
If however you use the chemical definition of “water” which is “A transparent fluid which forms the world’s streams, lakes, oceans and rain, and is the major constituent of the fluids of organisms” then you have to count the water contained in the urine (95% water), sweat (99% water) and water vapor produced by animals too. Animals do indeed produce more of the chemistry definition of water than they consume.
https://pubchem.ncbi.nlm.nih.gov/compound/water
https://www.dovemed.com/health-topics/focused-health-topics/urine-comprehensive-understanding-its-composition-function-and-health-significance
https://wellwisp.com/why-does-my-sweat-taste-like-water/
As one Ph.D. chemist to another I’d have expected you to use THAT definition in this discussion.
Well said.
Cheap Bastard already has written a very good response, but to add to your space angle, we do lose the ability to create water by sending rockets into space. Rocket fuel will usually contain either hydrogen outright or substances like kerosene, which contain hydrogen atoms. While burning the fuel creates water (of course), some of it is created outside of where Earth’s gravity is able to claw it back.
It’s a good thing we have no shortage of water, or hydrogen, on Earth any time soon.
Absolutely! That is another of the tiny leakages of water from the planet. And while we have no shortage of water, we do have a tremendous shortage of hydrogen in free form. Virtually all the hydrogen on earth is tied up in chemical compounds, which can be released through input of work and energy.
And while we have no overall shortage of water, we do have a shortage in many parts of the world of potable water. Of course, all water on earth is connected through the hydrologic cycle so conserving all forms of water (and producing potable water for our needs) is extremely important. As I pointed out in my response to Cheap Bastard, in this age where the public is increasingly ignorant of science, if people begin to believe that “animals create water”, they may be less likely to conserve the limited supply we have – “just raise more animals to create more water, bro!”. That, and the fact that animals are not net producers of water, is why I objected to your characterization of animals as “creating” water.
But the fact of the matter is that animals do create water, as we have clearly demonstrated, as a byproduct of metabolizing food. The question not if they create water (they do) but what they create the water from, which is food and oxygen. Both of which are provided, directly or indirectly, by plants during photosynthesis.
Guess what also creates water? You know already: burning fossil fuels. Burning one liter of petrol will create new water vapour, enough to make about a liter, give or take, of fluid water (you need to condense it of course).
That is a fact. That’s just how burning organic compounds works. Should people therefore think that burning fossil fuels is good for the environment because it creates water? Absolutely not.
we do lose the ability to create water by sending rockets into space. Rocket fuel will usually contain either hydrogen outright or substances like kerosene, which contain hydrogen atoms. While burning the fuel creates water (of course), some of it is created outside of where Earth’s gravity is able to claw it back.
Very little of what we send into space has escape velocity. Most of it falls back to earth, eventually. I would think that includes burned fuel.
Plants also emit water through their leaves into the air when they take in too much through their roots.
Maybe, but that water is not chemically processed at all. It came into the plant as water molecules, and it leaves the plant as the same water molecules. The water the plant has taken on and processed through photosynthesis is actually no longer water; it is now oxygen and biomass. The water has been chemically processed to no longer be water.
Not true. Many plants product proteins and water is a byproduct of protein synthesis. Sure, most of the water plants emit is unprocessed (like most of the water animals emit), but some of it has been chemically processed.
“Plants also emit water through their leaves into the air when they take in too much through their roots.” is what Jonathan wrote. I would suggest that water is just passed through to evaporate.
Incorrect. Most of the water is passed through (which is also true of animals) but some of the water is produced by chemical reactions to product proteins and other compounds.
In plants? Which destroy water during photosynthesis? Do you have a chemical reaction for a synthesis inside a plant that produces water? I have looked, but I haven’t been able to find any.
Yes indeed. Peptide bond synthesis is required for protein production, whether in plants or animals. This reaction produces (“creates” in your lingo) a water molecule.
Also, it may surprise you to learn that animals also use (“destroy”) water when breaking down proteins, among many other reactions going on in their systems. Animals are not net producers of water. They use far more water than they produce. Otherwise, they wouldn’t need to drink water constantly.
One reason why we animals need to drink is to stay cool. Animals emit a lot of energy, and sweating helps us do that. The water that is created is not nearly enough. That’s, for humans, probably the main reason, as we sweat a lot more than we are aware.
I have looked far and wide, and haven’t found any evidence that animals (among them humans) destroy water, on any large scale. None of the articles I have seen on synthesizing proteins, in animals or plans, have mentioned water going into this,
And I find it hard to believe, given that we already know that plants do destroy water on a giant scale to feed us all (6CO2 + 6H2O → C6H12O6 + 6O2 and all that jazz), somehow that loss of water has to be offset, and that is by us animals creating water.
As the saying goes, a little knowledge is a dangerous thing.
First, peptide bond synthesis is a requirement for protein production in organisms (animal or plant). Here’s a nice primer on the subject: https://byjus.com/jee/peptide-bond/. Peptide bond synthesis produces water. Water is so ubiquitous in biological chemical reactions that it is rarely called out in net chemical reactions for large systems, so you won’t see it represented most of the time.
Just as peptide synthesis produces water, peptide lysis uses water because it’s the reverse process: https://scienceinsider.blog/peptide-hydrolysis/.
What you seem to fail to grasp is that all biological systems are enormously complex and water is required for many different functions, physical, regulatory, solvent-related and as a chemical reactant or product.
Your simplistic characterization that “plants destroy water so animals must create it” is ignorant beyond belief for any adult human. You do realize that plants existed for billions of years before there were any animals, right? Do you know what else releases enormous amount of water back into the environment? The burning and decomposition of plant matter, which represents the vast majority of all biomass on the planet.
In fact, ALL animals combined comprise only 0.47% of all the biomass on the planet. Do you REALLY think that animals could make up for the deficit in water created by the nearly 83% of all biomass represented by plants? It’s ludicrous to think so. (https://www.earthdate.org/episodes/biomass-census)
Sure, animals and plants are teammates in the great circle of life, but the reality of that existence is far more complex than you seem to be able to grasp.
I do recognize that natural decay of biomass creates water. I mean, duh. Of course it does. So does burning biomass. So does burning most fossil fuels. Oxydizing biomatter creates water.
I have now learned that Peptide Hydrolysis destroys water. “This cleavage necessitates the incorporation of a water molecule (H₂O). The water molecule is cleaved, and its constituent parts are added to the resultant molecules.”
Well, yes, I did read on from there. So I learned that the presence of water is required for a lot of biochemical processes, but as far as I can tell so far water is not actually destroyed in these processes.
I mean, we have the very simple formula of 6CO₂ + 6H₂O + light energy → C₆H₁₂O₆ + 6CO₂ for photosynthesis and C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O + heat to describe how glucose created and burnt to feed us.
Of course I know that plants convert glucose into fats and proteins, but I have not been able to find how that would affect water creation or destruction.
Bring it on! I am seriously curious,
Sorry if I got a little salty, but you seem to have some degree of intelligence and your assertion that the tiny number of animals on the planet could offset the use of water by plants in photosynthesis is just such a ridiculous position to take! But perhaps you didn’t realize that animals are such a small percentage of life on this planet.
Anyway, thanks for your inquiry about production of water through protein and fat synthesis. I’ve already provided you with the chemical reactions necessary to produce proteins (the opposite of protein degradation), but I’ll repeat it here. It’s the reaction necessary to join two peptides, which is a necessary step in the synthesis of any protein in any biological system. I’ve represented the amino acid residues as A1 and A2, but of course they could be any two amino acids, even two of the same kind. It won’t be pretty like it was in the reference I linked above:
A1COO- + (+NH3)A2 –> A1CO-NHA2 + H2O.
Similarly, when fatty acids are produced in any biological system, a molecule of water is produced. That’s way too complex to try to represent here, but you can see it in this Wikipedia article: https://en.wikipedia.org/wiki/Fatty_acid_synthesis
When you consider that, on average, plant proteins consist of several hundred amino acids, and that several hundred water molecules were released during the production of each protein, then you can imagine that plants produce quite a lot of water through this one internal process, not to mention others. Of course animals produce water in the same ways. Both plants and animals use and produce and store a lot of water.
If you still insist on viewing the situation in a very simplistic manner, then you might consider that animals are net producers of CO2 (they don’t intake very much and they produce a lot), and that plants are net users of CO2. Or that animals are net users of oxygen and plants are net producers of oxygen. But the use and production of water is far too complex and nuanced to be reduced to such a simplistic syllogism as you suggested.
animas (including humans) create water!
So does (ironically) fire.
That depends on what you are burning, but generally speaking, yes, if you burn organic matter such as wood, petrol, diesel, natural gas etc., then water is created.
This is amazing! It looks great and the idea is delightful.
I am glad you made this and it is out in the world.